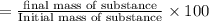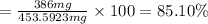Chemistry, 11.12.2019 23:31, Mypasswordishotdog11
Suppose an aspirin™ tablet contains 7 grains of the active ingredient, acetylsalicylic acid (1 grain = 64.7989 mg). if your tablet had a mass of 516 mg, calculate the percent recovery if you isolated 386 mg of acetylsalicylic acid in this experiment.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:00, thebasedgodchri
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1


Chemistry, 23.06.2019 00:50, trinityine
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
Do you know the correct answer?
Suppose an aspirin™ tablet contains 7 grains of the active ingredient, acetylsalicylic acid (1 grain...
Questions in other subjects:


Mathematics, 22.04.2021 01:00

Mathematics, 22.04.2021 01:00

Mathematics, 22.04.2021 01:00

Mathematics, 22.04.2021 01:00

Mathematics, 22.04.2021 01:00

History, 22.04.2021 01:00



Mathematics, 22.04.2021 01:00