Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, gonzalesalexiaouv1bg
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 16:30, danbelucio
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
Do you know the correct answer?
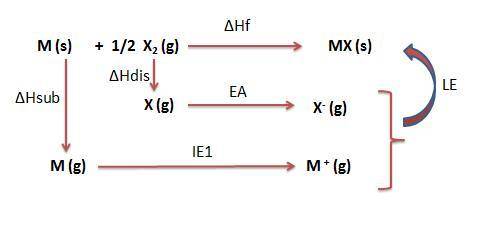
Calculate the lattice energy (kj/mol) of the hypothetical ionic compound mx from the information giv...
Questions in other subjects:




Mathematics, 11.03.2022 23:20

Mathematics, 11.03.2022 23:20


Mathematics, 11.03.2022 23:20