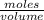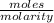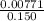Chemistry, 11.12.2019 22:31, ginger87771
What volume (in ml) of a 0.150 m hno 3 solution will completely react with 35.7 ml of a 0.108 m na 2 co 3 solution according to this balanced chemical equation?
na 2 co 3 ( a q ) + 2 hno 3 ( a q ) → 2 nano 3 ( a q ) + co 2 ( g ) + h 2 o ( l )

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, ayaanwaseem
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1
Do you know the correct answer?
What volume (in ml) of a 0.150 m hno 3 solution will completely react with 35.7 ml of a 0.108 m na 2...
Questions in other subjects:


Biology, 15.07.2019 15:00


Geography, 15.07.2019 15:00

Geography, 15.07.2019 15:00

Biology, 15.07.2019 15:00