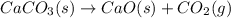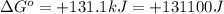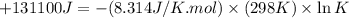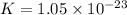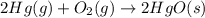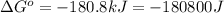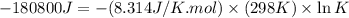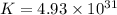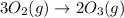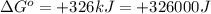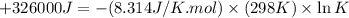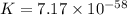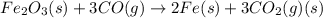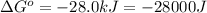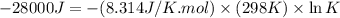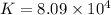Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chameleonsarelife
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 14:00, asanchez4292
What type of matter is made of only one kind of atom
Answers: 2

Chemistry, 22.06.2019 19:00, Jasoncookies23
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
Do you know the correct answer?
Which of the following reactions will have the largest value of k at 298 k? a) caco3(s) → cao(s) +...
Questions in other subjects:

Mathematics, 26.02.2021 05:20

Mathematics, 26.02.2021 05:20





Physics, 26.02.2021 05:20

Mathematics, 26.02.2021 05:20

Mathematics, 26.02.2021 05:20


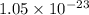
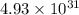
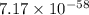
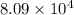
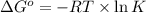
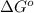 = standard Gibbs free energy
= standard Gibbs free energy