
Chemistry, 11.12.2019 20:31, brainist71
Consider the following equilibrium at 970 k for the dissociation of molecular iodine into atoms of iodine. i2(g) equilibrium reaction arrow 2 i(g); kc = 1.35 ✕ 10−3 suppose this reaction is initiated in a 3.7 l container with 0.063 mol i2 at 970 k. calculate the concentrations of i2 and i at equilibrium.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:30, alaf05160
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks. energy was destroyed inside the blocks. energy was absorbed into the blocks from outside the system. energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 17:20, banna01man
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2
Do you know the correct answer?
Consider the following equilibrium at 970 k for the dissociation of molecular iodine into atoms of i...
Questions in other subjects:




Mathematics, 15.07.2020 01:01



Mathematics, 15.07.2020 01:01




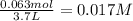
![Kc=1.35 \times 10^{-3} =\frac{[I]^{2} }{[I_{2}]} =\frac{(2x)^{2} }{(0.017-x)} \\4x^{2} +1.35 \times 10^{-3}x - 2.3 \times 10^{-5}](/tpl/images/0413/9700/35688.png)





