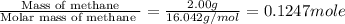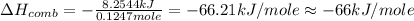Chemistry, 11.12.2019 19:31, wcraig1998
When 2.00 g of methane are burned in a bomb calorimeter, the change in temperature is 3.08°c. the heat capacity of the calorimeter is 2.68 kj/°c. the molar mass of methane is 16.042 g/mol. what is the approximate molar enthalpy of combustion of this substance?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, roseemariehunter12
When the earth was formed and cooled, why did nickel and iron end up in the center of the earth while basalt and granite ended up in the outer layers
Answers: 3


Chemistry, 23.06.2019 01:00, crysderria
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1
Do you know the correct answer?
When 2.00 g of methane are burned in a bomb calorimeter, the change in temperature is 3.08°c. the he...
Questions in other subjects:


Mathematics, 02.04.2020 16:52

Mathematics, 02.04.2020 16:52



Mathematics, 02.04.2020 16:53

Mathematics, 02.04.2020 16:53

History, 02.04.2020 16:53



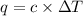
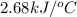
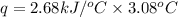
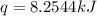
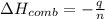
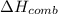 = enthalpy change = ?
= enthalpy change = ?