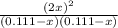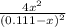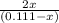Chemistry, 11.12.2019 19:31, ImBADatmath8743
The equilibrium constant for the reaction of fluorine gas with bromine gas at 300 k is 54.7 and the reaction is: br2(g) + f2(g) ⇔ 2 brf(g) what is the equilibrium concentration of fluorine if the initial concentrations of bromine and fluorine were 0.111 moles/liter in a sealed container and no product was present initially?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:50, duracohack
5. how can you decrease the pressure of a gas in a container without changing the volume of the gas?
Answers: 1

Chemistry, 22.06.2019 00:00, reeceslife481
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1


Chemistry, 22.06.2019 13:00, carlinryan
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2
Do you know the correct answer?
The equilibrium constant for the reaction of fluorine gas with bromine gas at 300 k is 54.7 and the...
Questions in other subjects:





English, 08.10.2021 08:40


Mathematics, 08.10.2021 08:40

Chemistry, 08.10.2021 08:40

Mathematics, 08.10.2021 08:40

Mathematics, 08.10.2021 08:40


![\frac{[BrF ]^{2} }{[ F_{2} ][Br_{2} ]}](/tpl/images/0413/8603/382cd.png)