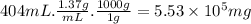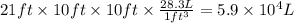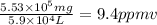Chemistry, 11.12.2019 18:31, serenityarts123
Aclumsy chemist drops a beaker containing 404 ml of a solvent (mw = 88.2, sg = 1.37) in a room measuring 21 ft x 10 ft x 10 ft. assuming that the contents of the beaker completely evaporate and fill the space, what is the resulting concentration in parts per million by volume (ppmv)? assume normal temperature and pressure (ntp), i. e., p = 1 atm, and t = 25 celsius. 1 ft3 = 28.3 l

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, sandersmakaylaovq5vu
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 12:40, jaylen2559
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1
Do you know the correct answer?
Aclumsy chemist drops a beaker containing 404 ml of a solvent (mw = 88.2, sg = 1.37) in a room measu...
Questions in other subjects:

Mathematics, 23.06.2020 10:57


Mathematics, 23.06.2020 10:57

English, 23.06.2020 10:57


Mathematics, 23.06.2020 10:57