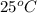Chemistry, 11.12.2019 18:31, lilquongohard
A100.0g sample of lead is heated to 100.0 oc (celsius) and is placed in a coffee cup calorimeter containing 150. g of water at 25.0 oc. after the metal cools, the final temperature of the metal and the water is 26.4 oc. calculate the specific heat capacity of lead from these experimental data, assuming that no heat escapes to the surroundings or is transferred to the calorimeter. specific heat of water = 4.184 j/g oc atomic mass pb = 207.2 g/mol calculate your answer in j/g oc with 3 significant figures, but enter the answer without units.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, letsbestupidcx2314
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 08:00, mariamakonteh31
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1
Do you know the correct answer?
A100.0g sample of lead is heated to 100.0 oc (celsius) and is placed in a coffee cup calorimeter con...
Questions in other subjects:

English, 03.02.2020 20:01


Mathematics, 03.02.2020 20:01

Mathematics, 03.02.2020 20:01


Social Studies, 03.02.2020 20:01

French, 03.02.2020 20:01

Physics, 03.02.2020 20:01


Mathematics, 03.02.2020 20:01


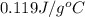


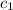 = specific heat of lead = ?
= specific heat of lead = ?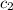 = specific heat of water =
= specific heat of water = 
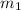 = mass of lead = 100.0 g
= mass of lead = 100.0 g = mass of water = 150.0 g
= mass of water = 150.0 g = final temperature =
= final temperature = 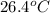
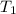 = initial temperature of lead =
= initial temperature of lead = 
 = initial temperature of water =
= initial temperature of water =