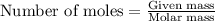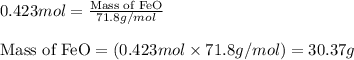Chemistry, 11.12.2019 05:31, nichelle2807
What mass of iron(ii) oxide must be used in the reaction given by the equation below to release 44.7 kj? 6feo(s) + o2(g) => 2fe3o4(s) δh° = -635 kj calculate your answer in g. enter it with two decimal places and no units.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:50, mckinleesmomp6qj1e
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Do you know the correct answer?
What mass of iron(ii) oxide must be used in the reaction given by the equation below to release 44.7...
Questions in other subjects:


Mathematics, 11.02.2021 14:00


Mathematics, 11.02.2021 14:00

Mathematics, 11.02.2021 14:00



Mathematics, 11.02.2021 14:00



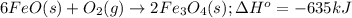
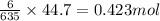 of iron (II) oxide is reacted.
of iron (II) oxide is reacted.