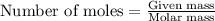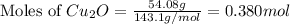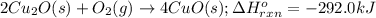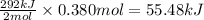Chemistry, 11.12.2019 05:31, Lydiac8715
The oxidation of copper(i) oxide, cu 2 o ( s ) , to copper(ii) oxide, cuo ( s ) , is an exothermic process. 2 cu 2 o ( s ) + o 2 ( g ) ⟶ 4 cuo ( s ) δ h ∘ rxn = − 292.0 kj mol calculate the energy released as heat when 54.08 g cu 2 o ( s ) undergo oxidation at constant pressure.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, mandy9386
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2


Chemistry, 22.06.2019 23:00, tovarclaudia055
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
Do you know the correct answer?
The oxidation of copper(i) oxide, cu 2 o ( s ) , to copper(ii) oxide, cuo ( s ) , is an exothermic p...
Questions in other subjects:

Biology, 12.02.2021 20:00




Mathematics, 12.02.2021 20:00

Health, 12.02.2021 20:00





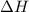 for the reaction will be -55.48 kJ
for the reaction will be -55.48 kJ