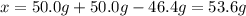Chemistry, 11.12.2019 03:31, kulvindar1984
Suppose 50.0g of silver nitrate is reacted with 50g of hydrochloric acid producing silver chloride and a mixture of other products. if the mass of the other products formed is 46.4 grams, how much silver chloride was produced?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 21:00, alwaysneedhelp84
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
Do you know the correct answer?
Suppose 50.0g of silver nitrate is reacted with 50g of hydrochloric acid producing silver chloride a...
Questions in other subjects:







Mathematics, 05.10.2020 15:01


Mathematics, 05.10.2020 15:01