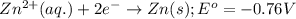Consider the following standard reduction potentials: zn2+(aq) + 2 e− latex: \longrightarrow⟶ zn(s) eo = −0.76 v mg2+(aq) + 2 e− latex: \longrightarrow⟶ mg(s) eo = −2.37 v ag+(aq) + e− latex: \longrightarrow⟶ ag(s) eo = +0.80 v which is the strongest oxidizing agent? group of answer choices

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, lindseyklewis1p56uvi
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 22:30, StupidFatChipmunk
What must be in balance for temperatures to remain constant?
Answers: 1

Chemistry, 22.06.2019 22:30, kiera2599
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
Do you know the correct answer?
Consider the following standard reduction potentials: zn2+(aq) + 2 e− latex: \longrightarrow⟶ zn(s...
Questions in other subjects:

Mathematics, 04.02.2021 23:20




History, 04.02.2021 23:20


Mathematics, 04.02.2021 23:20


Mathematics, 04.02.2021 23:20

English, 04.02.2021 23:20


 potential will always get reduced and will undergo reduction reaction easily.
potential will always get reduced and will undergo reduction reaction easily.