
Chemistry, 11.12.2019 02:31, sydthekid25
A1.0 g sample of hydrogen reacts completely with 19.0 g of fluorine to form a compound of hydrogen and fluorine. a. what is the percent by mass of each element in the compound? b. what mass of hydrogen would be present in a 50 g sample of this compound? c. justify your answer to b.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:20, Naysa150724
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 13:30, yasiroarafat12
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
Do you know the correct answer?
A1.0 g sample of hydrogen reacts completely with 19.0 g of fluorine to form a compound of hydrogen a...
Questions in other subjects:

English, 21.01.2021 16:30

Medicine, 21.01.2021 16:30

English, 21.01.2021 16:30

Mathematics, 21.01.2021 16:30


Biology, 21.01.2021 16:30

Chemistry, 21.01.2021 16:30

Mathematics, 21.01.2021 16:30


Biology, 21.01.2021 16:30




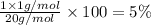

 of hydrogen atom.
of hydrogen atom.




