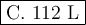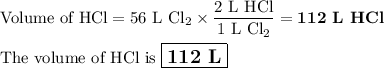Consider this reaction:
h2 (g) + cl2 (g) → 2 hcl (g)
how many liters of hcl are produce...

Chemistry, 11.12.2019 02:31, yashirachevalier
Consider this reaction:
h2 (g) + cl2 (g) → 2 hcl (g)
how many liters of hcl are produced when 56 l of chlorine are reacted with excess
hydrogen?
(one mole of any gas occupies 22.4 l under certain conditions of temperature and
pressure. assume those conditions for this question.)
a. 22.4l
b. 56 l
c. 112
d. 224 l

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:00, brandiwingard
What is the mass of phosphorous in a 51-kg person
Answers: 1

Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 22:30, kiera2599
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 26.05.2021 18:30

Advanced Placement (AP), 26.05.2021 18:30





Mathematics, 26.05.2021 18:30

Mathematics, 26.05.2021 18:30


Mathematics, 26.05.2021 18:30