
Consider four different samples: aqueous lif, molten lif, aqueous agf, and molten agf. current run through each sample produces one of the following products at the cathode: solid lithium, solid silver, or hydrogen gas. match each sample to its cathodic product. drag the appropriate items to their respective bins.

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 07:20, rscvsdfsrysas1857
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1
Do you know the correct answer?
Consider four different samples: aqueous lif, molten lif, aqueous agf, and molten agf. current run...
Questions in other subjects:

Mathematics, 10.10.2021 23:10

Mathematics, 10.10.2021 23:10

History, 10.10.2021 23:10

Mathematics, 10.10.2021 23:10


Advanced Placement (AP), 10.10.2021 23:10





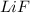 has water molecule, there will be competing will be two competing cations for reduction, while
has water molecule, there will be competing will be two competing cations for reduction, while 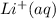 and
and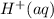 .However the reduction potential of
.However the reduction potential of  solid.
solid. contains water molecules.there will be competing will be two competing cations for reduction, while
contains water molecules.there will be competing will be two competing cations for reduction, while  and
and




