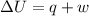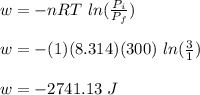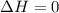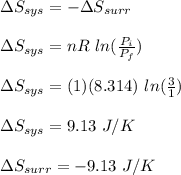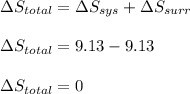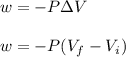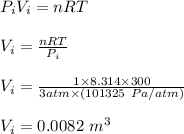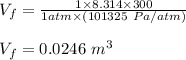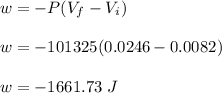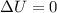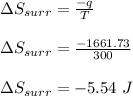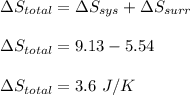Asample consisting of 1.00 mol of perfect gas molecules at 27 °c is expanded isothermally from an initial pressure of 3.00 atm to a final pressure of 1.00 atm in two ways: (a) reversibly, and (b) against a constant external pressure of 1.00 atm. evaluate q, w, δu, δh, δs, δssurr, and δstot in each case.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, maddynichole2017
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 06:00, coopera1744
Find the mass in grams of 1.37x1020 particles of h3po4
Answers: 2

Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3
Do you know the correct answer?
Asample consisting of 1.00 mol of perfect gas molecules at 27 °c is expanded isothermally from an in...
Questions in other subjects:



Mathematics, 12.07.2019 16:00

Mathematics, 12.07.2019 16:00


Mathematics, 12.07.2019 16:00


Computers and Technology, 12.07.2019 16:00


Mathematics, 12.07.2019 16:00


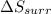 is -9.13 J/K, the entropy change of the system,
is -9.13 J/K, the entropy change of the system, 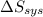 is 9.13 J/K, and the total entropy change,
is 9.13 J/K, and the total entropy change, 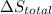 is 0.
is 0.