Chemistry, 10.12.2019 19:31, cdjeter12oxoait
Calculate the enthalpy of reaction for 2co + o2 → 2co2. given the following bond energies:
be(c≡o) = 1074 kj/mol
be(o≡o) = 499 kj/mol
be(c≡o) = 802 kj/mol

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, NorbxrtThaG
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 13:00, wbrandi118
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 23.06.2019 03:00, kdcloyd3362
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3
Do you know the correct answer?
Calculate the enthalpy of reaction for 2co + o2 → 2co2. given the following bond energies:
b...
b...
Questions in other subjects:

Mathematics, 21.04.2020 02:01


Mathematics, 21.04.2020 02:01

Mathematics, 21.04.2020 02:01

Business, 21.04.2020 02:01


English, 21.04.2020 02:01

Mathematics, 21.04.2020 02:01

Chemistry, 21.04.2020 02:01


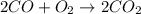
![\Delta H=[(2\times B.E_{C\equiv O})+(1\times B.E_{O\equiv O})]-[2\times B.E_{C=O}]](/tpl/images/0412/0179/8905d.png)
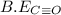 = 1074 kJ/mol
= 1074 kJ/mol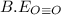 = 499 kJ/mol
= 499 kJ/mol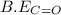 = 802 kJ/mol
= 802 kJ/mol![\Delta H=[(2\times 1074kJ/mol)+(1\times 499kJ/mol)]-[2\times 802kJ/mol]](/tpl/images/0412/0179/c40d4.png)