

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:10, cefindley14
Which statement is true about the part of the electromagnetic spectrum that human eyes can detect? it contains only the colors of the rainbow and television waves. o it is divided into seven ranges of wavelengths. it contains ultraviolet, visible, and infrared light. it is divided into nine ranges of wavelengths.
Answers: 2

Chemistry, 22.06.2019 12:10, yootmytoot
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution. calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1
Do you know the correct answer?
What is the excess reactant for the following reaction given we have 3.4 moles of ca(no3)2 and 2.4 m...
Questions in other subjects:

Mathematics, 05.09.2019 21:30



World Languages, 05.09.2019 21:30



Physics, 05.09.2019 21:30

English, 05.09.2019 21:30

History, 05.09.2019 21:30


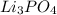
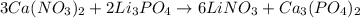
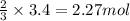 of lithium phosphate
of lithium phosphate



