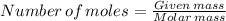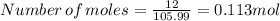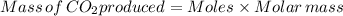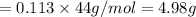Chemistry, 10.12.2019 10:31, tyaslovell
Suppose you used excess sulfuric acid in all the flasks. how many moles of co2would be released in flask 7? the molar mass of sodium carbonate is 105.99 g/mol.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, elizabethprasad2
How many grams of n2h4 will be consumed by 23 g of n2o4
Answers: 1


Chemistry, 22.06.2019 13:00, carlinryan
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
Do you know the correct answer?
Suppose you used excess sulfuric acid in all the flasks. how many moles of co2would be released in f...
Questions in other subjects:

Mathematics, 02.06.2021 01:00


Health, 02.06.2021 01:00



Mathematics, 02.06.2021 01:00

Biology, 02.06.2021 01:00


Mathematics, 02.06.2021 01:00