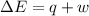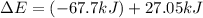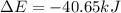Chemistry, 10.12.2019 06:31, wendelkristen
Consider a balloon filled with helium at the following conditions. 313 g he 1.00 atm 1910. l molar heat capacity = 20.8 j/degree c middot mol the temperature of this balloon is decreased by 41.6 degree c as the volume decreases to 1643 l with the pressure remaining constant. determine q, w, and delta e (in kj) for the compression of the balloon.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, asanchez4292
What type of matter is made of only one kind of atom
Answers: 2

Chemistry, 22.06.2019 21:00, andrethisman88
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 23.06.2019 04:31, 1Angel2Got3Brains
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1
Do you know the correct answer?
Consider a balloon filled with helium at the following conditions. 313 g he 1.00 atm 1910. l molar h...
Questions in other subjects:


Mathematics, 11.03.2021 14:00

Mathematics, 11.03.2021 14:00

Spanish, 11.03.2021 14:00

Mathematics, 11.03.2021 14:00


Mathematics, 11.03.2021 14:00

Mathematics, 11.03.2021 14:00


Mathematics, 11.03.2021 14:00


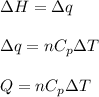
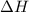 = change in enthalpy energy
= change in enthalpy energy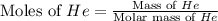
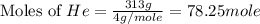
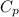 = heat capacity at constant pressure =
= heat capacity at constant pressure = 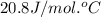
 = change in temperature =
= change in temperature = 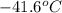
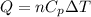
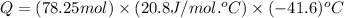
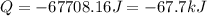
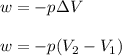
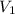 = initial volume = 1910 L
= initial volume = 1910 L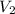 = final volume = 1643 L
= final volume = 1643 L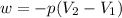
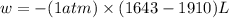
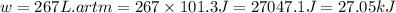
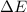 of the gas.
of the gas.