
Chemistry, 10.12.2019 05:31, kimberlyhansen92
Acertain liquid x has a normal boiling point of 108.30 °c and a boiling point elevation constant kb=1.07 °c kg/mol. a solution is prepared by dissolving some ammonium sulfate (nh4)2 so4 in 600. g of x. this solution boils at 109.7 °c. calculate the mass of (nh4)2 so4 that was dissolved. be sure your answer is rounded to the correct number of significant digits.

Answers: 1
Other questions on the subject: Chemistry



Do you know the correct answer?
Acertain liquid x has a normal boiling point of 108.30 °c and a boiling point elevation constant kb=...
Questions in other subjects:





History, 18.05.2021 23:50

Business, 18.05.2021 23:50

Business, 18.05.2021 23:50

Mathematics, 18.05.2021 23:50

Social Studies, 18.05.2021 23:50


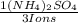 = 0,262 moles of (NH₄)₂SO₄
= 0,262 moles of (NH₄)₂SO₄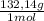 = 34,6g of (NH₄)₂SO₄
= 34,6g of (NH₄)₂SO₄



