Chemistry, 10.12.2019 05:31, star030616
Asample of calcium carbonate (caco3) is known to contain some impurities. it is found that calcium makes up 11% of the entire mass of the sample. all of the calcium comes from the caco3 compound. find the mass percent of caco3 in the sample. set up a ratio and solve.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, slugmilk1090
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 10:10, veronica022
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 20:00, bettybales1986
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2
Do you know the correct answer?
Asample of calcium carbonate (caco3) is known to contain some impurities. it is found that calcium m...
Questions in other subjects:

Mathematics, 12.05.2021 21:10

Mathematics, 12.05.2021 21:10


Mathematics, 12.05.2021 21:10

World Languages, 12.05.2021 21:10

Mathematics, 12.05.2021 21:10




Chemistry, 12.05.2021 21:10


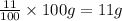
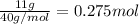
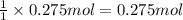 calcium carbonate
calcium carbonate