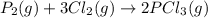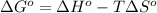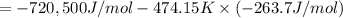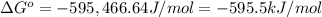Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, nana54muller
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 10:00, youngchapo813p8d9u1
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3


Chemistry, 22.06.2019 11:00, justarando
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3
Do you know the correct answer?
The value of δg∘ at 201.0∘c for the formation of phosphorous trichloride from its constituent elemen...
Questions in other subjects:


Physics, 09.12.2019 17:31


English, 09.12.2019 17:31


History, 09.12.2019 17:31

Social Studies, 09.12.2019 17:31



Mathematics, 09.12.2019 17:31