

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 17:30, rollercoasterbuddies
Why is the melting of ice a physical change ?
Answers: 1

Chemistry, 22.06.2019 23:00, jolainjoseph01998
What element has similar physical and chemical properties as boron.
Answers: 1
Do you know the correct answer?
Amixture of n2, o2, and ar has mole fractions of 0.25, 0.65, and 0.10, respectively. what is the pre...
Questions in other subjects:



Mathematics, 15.07.2019 00:10

Mathematics, 15.07.2019 00:10

Mathematics, 15.07.2019 00:10


Mathematics, 15.07.2019 00:10

History, 15.07.2019 00:10







 = total pressure = 3.9 bar
= total pressure = 3.9 bar  = partial pressure of nitrogen gas
= partial pressure of nitrogen gas 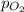 = partial pressure of oxygen gas
= partial pressure of oxygen gas 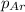 = partial pressure of argon gases
= partial pressure of argon gases  = Mole fraction of nitrogen gas = 0.25
= Mole fraction of nitrogen gas = 0.25 = Mole fraction of oxygen gas = 0.65
= Mole fraction of oxygen gas = 0.65 = Mole fraction of argon gases = 0.10
= Mole fraction of argon gases = 0.10







