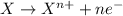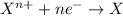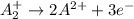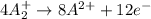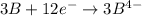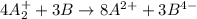Chemistry, 10.12.2019 04:31, summerhumphries3
Given two half reactions as follows: a2+ → 2 a2+ + 3 e− 4 e− + b → b4− what would you multiply each half-reaction by, to cancel out the electrons?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:30, erikloza12pdidtx
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 17:40, aaliyahthomas37
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 20:00, Chynadoll94
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
Do you know the correct answer?
Given two half reactions as follows: a2+ → 2 a2+ + 3 e− 4 e− + b → b4− what would you multiply each...
Questions in other subjects:




Social Studies, 04.10.2021 23:20

Mathematics, 04.10.2021 23:20

Physics, 04.10.2021 23:20


Mathematics, 04.10.2021 23:20

Mathematics, 04.10.2021 23:20