
Chemistry, 10.12.2019 04:31, sbudlove2838
Gfind the ∆hrxn for the reaction: 3c(s)+4h2(g) →c3h8(g) using these reactions with known ∆h’s: c3h8(g) + 5o2(g) → 3co2(g) + 4h2o(g) ∆h = −2043 kj c(s) + o2(g) → co2(g) ∆h = −393.5 kj 2h2(g) + o2(g) → 2h2o(g) ∆h = −483.6 kj

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:30, kaliloabousjbf
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3


Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
Do you know the correct answer?
Gfind the ∆hrxn for the reaction: 3c(s)+4h2(g) →c3h8(g) using these reactions with known ∆h’s: c3h...
Questions in other subjects:

Biology, 26.10.2020 22:40


Mathematics, 26.10.2020 22:40



History, 26.10.2020 22:40

Spanish, 26.10.2020 22:40

Biology, 26.10.2020 22:40

Mathematics, 26.10.2020 22:40

Mathematics, 26.10.2020 22:40


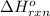 for the reaction is -104.7 kJ.
for the reaction is -104.7 kJ.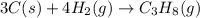
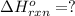
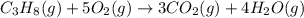
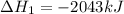
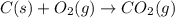
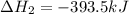 ( × 3)
( × 3) 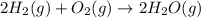
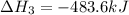 ( × 2)
( × 2) ![\Delta H^o_{rxn}=[1\times (-\Delta H_1)]+[3\times \Delta H_2]+[2\times \Delta H_3]](/tpl/images/0411/2485/b4dbe.png)
![\Delta H^o_{rxn}=[(1\times (-(-2043))+(3\times (-393.5))+(2\times (-483.6))]=-104.7kJ](/tpl/images/0411/2485/eafbb.png)




