
Chemistry, 10.12.2019 02:31, Maelynne8515
Consider the reaction, cl2 + h2s => 2 hcl + s, which is found to be first order in cl2. which step of the proposed mechanism must be slow in order to agree with this rate law? cl2 => 2 cl cl + h2s => hcl + hs cl + hs => hcl + s
a. only 3
b. only 2
c. 1
d. either 2 or 3

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, itzyagirlshy
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 22.06.2019 06:00, mapoohdoll
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 13:00, naomicervero
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3
Do you know the correct answer?
Consider the reaction, cl2 + h2s => 2 hcl + s, which is found to be first order in cl2. which st...
Questions in other subjects:

History, 23.03.2021 02:00

Mathematics, 23.03.2021 02:00

Mathematics, 23.03.2021 02:00


History, 23.03.2021 02:00

Mathematics, 23.03.2021 02:00

Mathematics, 23.03.2021 02:00

Mathematics, 23.03.2021 02:00



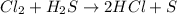
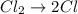
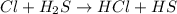
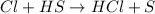
![rate=k[Cl_{2}]^\frac{1}{2}[H_{2}S]](/tpl/images/0410/9890/7d770.png)




