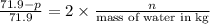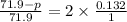Asolution of sodium chloride in water has a vapor pressure of 17.5 torr at 25°c. what is the mole fraction of nacl solute particles in this solution? what would be the vapor pressure of this solution at 45°c? the vapor pressure of pure water is 23.8 torr at 25°c and 71.9 torr at 45°c and assume sodium chloride exists as na⁺ and cl⁻ ions in solution.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:30, sbush1412
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u. s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 19:00, andrecoral105
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 23.06.2019 03:50, lindseyklewis1p56uvi
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
Do you know the correct answer?
Asolution of sodium chloride in water has a vapor pressure of 17.5 torr at 25°c. what is the mole fr...
Questions in other subjects:





Mathematics, 17.12.2021 01:10

English, 17.12.2021 01:10





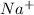 and
and 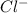 in solution. Therefore, Van't Hoff factor (i) will be equal to 2.
in solution. Therefore, Van't Hoff factor (i) will be equal to 2.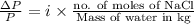
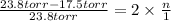
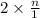
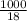
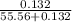
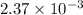
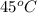 , the vapor pressure will be calculated as follows.
, the vapor pressure will be calculated as follows.