
Chemistry, 10.12.2019 01:31, bonetanne7171
Consider a solution containing 3.54 mm of an analyte, x, and 1.23 mm of a standard, s. upon chromatographic separation of the solution peak areas for x and s are 3275 and 10829, respectively. determine the response factor for x relative to s. f=to determine the concentration of x in an unknown solution, 1.00 ml of 8.27 mm s was added to 5.00 ml of the unknown x solution and the mixture was diluted to 10.0 ml. after chromatographic separation, this solution gave peak areas of 5389 and 4627 for x and s, respectively. determine the concentration of s in the 10.0 ml solution.[s]=determine the concentration of x in the 10.0 ml solution.[x]=determine the concentration of x in the unknown solution.[x]=

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Kaylinne1181
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 05:00, Angelanova69134
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 14:30, KennyOaks6230
Which of the following units is not an official si unit
Answers: 1

Do you know the correct answer?
Consider a solution containing 3.54 mm of an analyte, x, and 1.23 mm of a standard, s. upon chromato...
Questions in other subjects:




Health, 13.10.2020 14:01

Arts, 13.10.2020 14:01



Mathematics, 13.10.2020 14:01


History, 13.10.2020 14:01


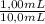 = 0,827mM
= 0,827mM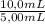 = 18,3mM
= 18,3mM



