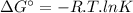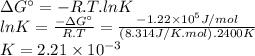Chemistry, 10.12.2019 01:31, mariahdelossantos031
The free energy change δgt at 2400 k is equal to 1.22 x 10^5j/mol. calculate the equilibrium constant at 2400 k. express your answer numerically.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 22:00, genyjoannerubiera
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1
Do you know the correct answer?
The free energy change δgt at 2400 k is equal to 1.22 x 10^5j/mol. calculate the equilibrium constan...
Questions in other subjects:




Biology, 28.01.2020 20:58



History, 28.01.2020 20:58


Mathematics, 28.01.2020 20:58

English, 28.01.2020 20:58