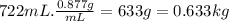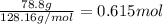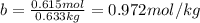Chemistry, 10.12.2019 01:31, erinwebsterrr
Determine the freezing point of a solution that contains 78.8 g of naphthalene (c10h8, molar mass = 128.16 g/mol) dissolved in 722 ml of benzene (d = 0.877 g/ml). pure benzene has a melting point of 5.50°c and a freezing point depression constant of 4.90°c/m.0.74°c4.76°c4.17°c1.68°c1. 33°c

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, brookekolmetz
How many orbitals does the p sub shell container
Answers: 3

Chemistry, 21.06.2019 23:00, fespinoza019
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 17:30, rollercoasterbuddies
Why is the melting of ice a physical change ?
Answers: 1
Do you know the correct answer?
Determine the freezing point of a solution that contains 78.8 g of naphthalene (c10h8, molar mass =...
Questions in other subjects:




Physics, 26.02.2020 17:25

Biology, 26.02.2020 17:25



History, 26.02.2020 17:25

Biology, 26.02.2020 17:25