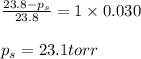Chemistry, 10.12.2019 01:31, romaguera06
Determine the vapor pressure of a solution at 25°c that contains 76.6 g of glucose (c6h12o6) in 250.0 ml of water. the vapor pressure of pure water at 25°c is 23.8 torr.70.8 torr7.29 torr72.9 torr22.9 torr23.1 torr

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:00, cooljariel11
Give more examples of this type of heat transfer:
Answers: 1

Chemistry, 23.06.2019 03:50, timothymoles
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1

Chemistry, 23.06.2019 11:30, elpeke102p73fz3
Jenny places a strip of ph paper into a solution. when she removes the ph paper, it has turned yellow-green. what should jenny do next to determine the ph of her solution? a. use a different testing method because the ph paper should not change colors b. place the ph paper into a machine that reads the ph of the solution c. compare the ph paper's color with the color of ph paper from another solution d. compare the ph paper's color with a chart of colors and ph ranges
Answers: 1
Do you know the correct answer?
Determine the vapor pressure of a solution at 25°c that contains 76.6 g of glucose (c6h12o6) in 250....
Questions in other subjects:

Mathematics, 27.09.2021 08:00

Mathematics, 27.09.2021 08:00

History, 27.09.2021 08:00


Social Studies, 27.09.2021 08:00

English, 27.09.2021 08:00





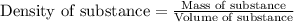
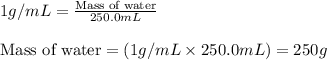
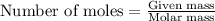 .....(1)
.....(1)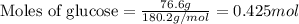
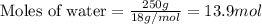
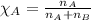
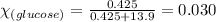
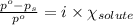
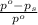 = relative lowering in vapor pressure
= relative lowering in vapor pressure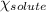 = mole fraction of solute = 0.030
= mole fraction of solute = 0.030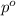 = vapor pressure of pure water = 23.8 torr
= vapor pressure of pure water = 23.8 torr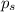 = vapor pressure of solution = ?
= vapor pressure of solution = ?