
Chemistry, 09.12.2019 22:31, davidaagurto
Predict the sign of the entropy change, delta s, for each of the following reactions: the signs are either going to be pos or negativea) pb^2+(aq) + 2cl-(aq) > pbcl2(s)b) caco3(s) > cao(s) + co2 (g)c) 2nh3(g) > n2(g) + 3h2(g)d) p4(g) + 5o2(g) > p4o10(s)e) c4h8(g) + 6o2(g) > 4co2(g) + 4h2o(g)f) i2(s) > i2(g)

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1


Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
Do you know the correct answer?
Predict the sign of the entropy change, delta s, for each of the following reactions: the signs are...
Questions in other subjects:

History, 04.12.2020 06:30


Mathematics, 04.12.2020 06:30

Chemistry, 04.12.2020 06:30

English, 04.12.2020 06:30

Mathematics, 04.12.2020 06:30

Mathematics, 04.12.2020 06:30

Mathematics, 04.12.2020 06:30

English, 04.12.2020 06:30


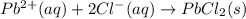 : negative
: negative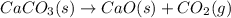 : positive
: positive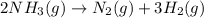 : positive.
: positive.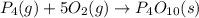 : negative
: negative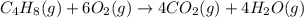 : positive.
: positive.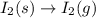 : positive.
: positive.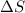 is positive when randomness increases and
is positive when randomness increases and 



