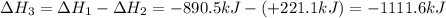Chemistry, 09.12.2019 22:31, santos200154
Given the reactions, x ( s ) + 1 2 o 2 ( g ) ⟶ xo ( s ) δ h = − 890.5 kj xco 3 ( s ) ⟶ xo ( s ) + co 2 ( g ) δ h = + 221.1 kj what is δ h for this reaction? x ( s ) + 1 2 o 2 ( g ) + co 2 ( g ) ⟶ xco 3 ( s )

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, pandasarecute53
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Do you know the correct answer?
Given the reactions, x ( s ) + 1 2 o 2 ( g ) ⟶ xo ( s ) δ h = − 890.5 kj xco 3 ( s ) ⟶ xo ( s ) + co...
Questions in other subjects:

Arts, 05.05.2020 06:41

Mathematics, 05.05.2020 06:41





Mathematics, 05.05.2020 06:41





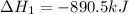 (1)
(1)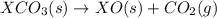
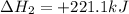 (2)
(2)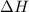 for the following reaction i.e,
for the following reaction i.e,
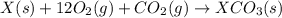
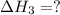 (3)
(3)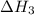 for the reaction will be:
for the reaction will be: