

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:20, montanolumpuy
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d. lytic
Answers: 1

Chemistry, 22.06.2019 23:00, poolwaterisgross
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 22.06.2019 23:30, billybob8514
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1

Chemistry, 23.06.2019 03:00, kuehlthau03
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1
Do you know the correct answer?
Sodium carbonate can be made by heating sodium bicarbonate: 2nahco3(s) → na2co3(s) + co2(g) + h2o(g...
Questions in other subjects:


English, 20.06.2021 01:10

Mathematics, 20.06.2021 01:10

Mathematics, 20.06.2021 01:10

English, 20.06.2021 01:10






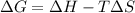
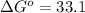
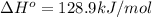
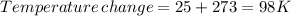
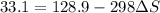
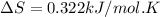
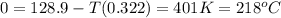
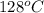 minimum temperature will the reaction become spontaneous.
minimum temperature will the reaction become spontaneous.



