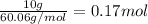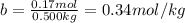Chemistry, 09.12.2019 20:31, alex12everett
The molal freezing point depression constant =kf6.19·°c·kgmol−1 for a certain substance x . when 10.g of urea nh22co are dissolved in 500.g of x , the solution freezes at −6.8°c . calculate the freezing point of pure x . be sure your answer has the correct number of significant digits.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 03:40, 19thomasar
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3
Do you know the correct answer?
The molal freezing point depression constant =kf6.19·°c·kgmol−1 for a certain substance x . when 10....
Questions in other subjects:


Mathematics, 09.11.2020 18:00


Mathematics, 09.11.2020 18:00

Mathematics, 09.11.2020 18:00



Engineering, 09.11.2020 18:00