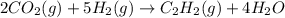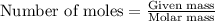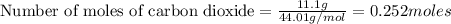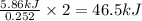Chemistry, 09.12.2019 20:31, poptropic9207
The reaction of carbon dioxide(g) with hydrogen(g) to form acetylene(g) (c2h2) and water(g) proceeds as follows: 2 co2(g) + 5 h2(g) c2h2(g) + 4 h2o(g) when 11.1 grams of co2(g) react with sufficient h2(g) , 5.86 kj of energy are absorbed . what is the value of h for the chemical equation given? δhrxn = kj

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, JuniperGalaxy
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1


Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1
Do you know the correct answer?
The reaction of carbon dioxide(g) with hydrogen(g) to form acetylene(g) (c2h2) and water(g) proceeds...
Questions in other subjects:

Geography, 17.07.2019 03:30

History, 17.07.2019 03:30

Mathematics, 17.07.2019 03:30

Biology, 17.07.2019 03:30

Mathematics, 17.07.2019 03:30

Mathematics, 17.07.2019 03:30

Social Studies, 17.07.2019 03:30

Health, 17.07.2019 03:30