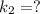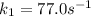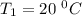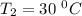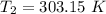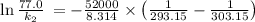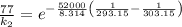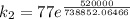Chemistry, 09.12.2019 20:31, brenda0113hernandez
The rate constant for a certain chemical reaction is 77.0 s-1 at a temperature of 20.0°c. if the activation energy for this reaction is 52. kj/mole, what is the rate constant at a temperature of 30.0°c?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, hdjsjfjruejchhehd
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 20:00, aksambo4707
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 22.06.2019 23:00, Mw3spartan17
What extra step distinguishes fermentation from glycolysis
Answers: 1
Do you know the correct answer?
The rate constant for a certain chemical reaction is 77.0 s-1 at a temperature of 20.0°c. if the act...
Questions in other subjects:






Health, 27.08.2019 08:00

Social Studies, 27.08.2019 08:00

Mathematics, 27.08.2019 08:00


Social Studies, 27.08.2019 08:00


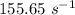 is the rate constant at a temperature of 30.0 °C.
is the rate constant at a temperature of 30.0 °C.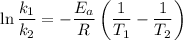
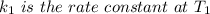
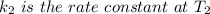
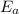 is the activation energy
is the activation energy