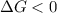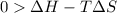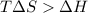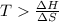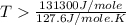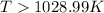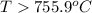Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, alvaradolm6853
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 22:30, lanashanabJHsbd1099
Who discovered a pattern to the elements in 1869?
Answers: 1
Do you know the correct answer?
For the reaction c(s)+h2o(g)→co2(g)+h2(g) δh∘=131.3kj/mol and δs∘=127.6j/k⋅mol at 298k. at temperatu...
Questions in other subjects:






Mathematics, 26.03.2020 21:07


Mathematics, 26.03.2020 21:07



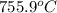 this reaction is spontaneous under standard conditions.
this reaction is spontaneous under standard conditions.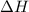 = 131.3 KJ/mole = 131300 J/mole
= 131.3 KJ/mole = 131300 J/mole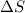 = 127.6 J/mole.K
= 127.6 J/mole.K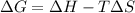
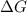 is negative or we can say that the value of
is negative or we can say that the value of