
Chemistry, 09.12.2019 18:31, pooooooooyp4286
When nh3(g) reacts with o2(g), the products of the combustion are no(g) and h2o(g). what volume of o2(g) is required to react with 3.00 ml of nh3(g)? assume that all gases are at the same temperature and pressure.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lakenyagillard79
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2


Chemistry, 22.06.2019 09:50, bridgetosanders
What are four significant sources of ghgs that come from wostem washington?
Answers: 2
Do you know the correct answer?
When nh3(g) reacts with o2(g), the products of the combustion are no(g) and h2o(g). what volume of o...
Questions in other subjects:



History, 20.07.2019 07:00

Mathematics, 20.07.2019 07:00

Physics, 20.07.2019 07:00

History, 20.07.2019 07:00


English, 20.07.2019 07:00

Mathematics, 20.07.2019 07:00

History, 20.07.2019 07:00


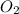 required is:- 3.75 mL
required is:- 3.75 mL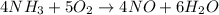
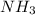 is required to react with 5 mL of
is required to react with 5 mL of 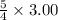 mL of
mL of 



