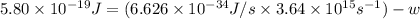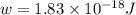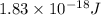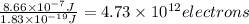Chemistry, 09.12.2019 18:31, gajdmaciej9502
When a metal was exposed to light at a frequency of 3.64× 1015 s–1, electrons were emitted with a kinetic energy of 5.80× 10–19 j. what is the maximum number of electrons that could be ejected from this metal by a burst of light (at some other frequency) with a total energy of 8.66× 10–7 j?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, cynthiagutierrez65
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 21.06.2019 22:00, carlybeavers50
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 02:50, Jerrikasmith28
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Do you know the correct answer?
When a metal was exposed to light at a frequency of 3.64× 1015 s–1, electrons were emitted with a ki...
Questions in other subjects:

Mathematics, 28.05.2021 16:10


Health, 28.05.2021 16:10

Mathematics, 28.05.2021 16:10




Advanced Placement (AP), 28.05.2021 16:10

Physics, 28.05.2021 16:10

Mathematics, 28.05.2021 16:10


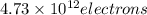
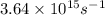
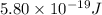
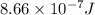
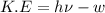
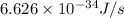
 = frequency
= frequency