425 ml of neon gas at -12°c and 788 mmhg
expands into a 2.40 l container while the
press...


Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, armahoney8566
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 14:50, rebeccamckellpidge
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 23.06.2019 00:20, destromero
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
Do you know the correct answer?
Questions in other subjects:

History, 14.07.2019 14:10

Mathematics, 14.07.2019 14:10

History, 14.07.2019 14:10


Mathematics, 14.07.2019 14:10

English, 14.07.2019 14:10




Mathematics, 14.07.2019 14:10


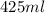
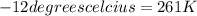
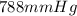
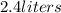
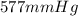
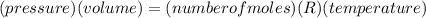
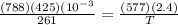
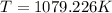 = 806.226 degrees celcius
= 806.226 degrees celcius



