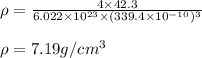Chemistry, 22.08.2019 08:30, domenica19
The atomic radius of metal x is 1.20 × 102 picometers (pm) and a crystal of metal x has a unit cell that is face-centered cubic. calculate the density of metal x? (atomic weight = 42.3 g/mol)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:10, tishfaco5000
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 08:30, aydenmasonc
Which statement describes james chadwick’s discovery.
Answers: 2

Chemistry, 22.06.2019 23:00, SophieCasey
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1

Chemistry, 23.06.2019 01:50, kayleebueno
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
Do you know the correct answer?
The atomic radius of metal x is 1.20 × 102 picometers (pm) and a crystal of metal x has a unit cell...
Questions in other subjects:

Mathematics, 18.12.2019 00:31










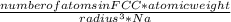 . Substituting the given, the density is 162.69 g/cm3.
. Substituting the given, the density is 162.69 g/cm3. 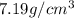
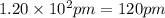
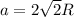
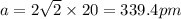
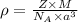
 = density
= density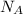 = Avogadro's number =
= Avogadro's number = 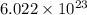
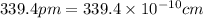 (Conversion factor:
(Conversion factor: 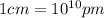 )
)