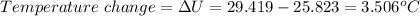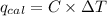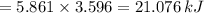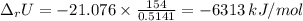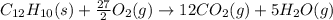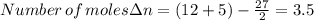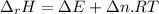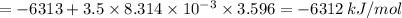Chemistry, 08.12.2019 20:31, gabrielaelisa224
When 0.5141 g of biphenyl (c12h10) undergoes combustion in a bomb calorimeter, the temperature rises from 25.823 °c to 29.419 °c. find δru and δrh for the combustion of biphenyl in kj mol−1 at 298 k. the heat capacity of the bomb calorimeter, determined in a separate experiment, is 5.861 kj °c−1.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2


Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 21:50, SoccerAllStar2
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
Do you know the correct answer?
When 0.5141 g of biphenyl (c12h10) undergoes combustion in a bomb calorimeter, the temperature rises...
Questions in other subjects:

Mathematics, 02.04.2021 19:10


Mathematics, 02.04.2021 19:10

Social Studies, 02.04.2021 19:10


Health, 02.04.2021 19:10




Mathematics, 02.04.2021 19:10


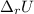 of the reaction is -6313 kJ/mol
of the reaction is -6313 kJ/mol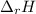 of the reaction is -6312 kJ/mol
of the reaction is -6312 kJ/mol