
Chemistry, 01.12.2019 00:31, deaishaajennings123
Calculate the approximate enthalpy change, δhrxn, for the combustion of methane: ch4+2o2→2h2o+co2 δhrxn from a given table: ch4 = 1656 kj/mol o2 = 498 kj/mol h2o = 928 kj/mol co2 = 1598 kj/mol?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, Cnolteb5663
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b. slope c. benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 21:50, namoralessimon03
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1

Chemistry, 22.06.2019 23:30, znewkirk4741
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
Do you know the correct answer?
Calculate the approximate enthalpy change, δhrxn, for the combustion of methane: ch4+2o2→2h2o+co2 δ...
Questions in other subjects:

Geography, 27.04.2021 23:00

Mathematics, 27.04.2021 23:00

Computers and Technology, 27.04.2021 23:00

Social Studies, 27.04.2021 23:00

Mathematics, 27.04.2021 23:00

Mathematics, 27.04.2021 23:00

History, 27.04.2021 23:00


English, 27.04.2021 23:00

Mathematics, 27.04.2021 23:00


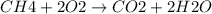
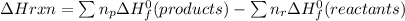
![\Delta Hrxn = [2\Delta H_{f}^{0}(H2O)+1\Delta H_{f}^{0}(CO2)]-[1\Delta H_{f}^{0}(CH4)+ 2\Delta H_{f}^{0}(O2)]](/tpl/images/0397/7957/b0337.png)




