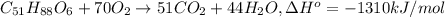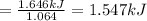Chemistry, 07.12.2019 06:31, school4life110
One possible use for the cooking fat left over after making french fries is to burn it as fuel.
formula = c(51)h(88)o(6)
density = 0.94 g/ml
delta h^degree = - 1310 kj/mol.
write a balanced equation of the combustion of cooking fat. use the data above to calculate the amount of energy released (in kilojoules per milliliter) from the combustion of cooking fat:

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:20, camillexv2668
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1


Chemistry, 22.06.2019 16:10, nauticatyson9
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 16:50, struckedblazing
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
Do you know the correct answer?
One possible use for the cooking fat left over after making french fries is to burn it as fuel.
Questions in other subjects:


History, 10.06.2021 20:30




Biology, 10.06.2021 20:30

Mathematics, 10.06.2021 20:30

Mathematics, 10.06.2021 20:30


Mathematics, 10.06.2021 20:30