

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, alexandroperez13
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3


Chemistry, 22.06.2019 07:00, maryjane8872
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1
Do you know the correct answer?
An atom (not a hydrogen atom) absorbs a photon whose associated wavelength is 300 nm and then immedi...
Questions in other subjects:

Mathematics, 31.03.2021 02:00

Mathematics, 31.03.2021 02:00

Mathematics, 31.03.2021 02:00


Social Studies, 31.03.2021 02:00

Mathematics, 31.03.2021 02:00


Physics, 31.03.2021 02:00



 - 1/λ
- 1/λ )
)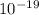 J.
J.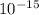 eVs
eVs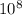 m/s
m/s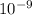 m
m )
) 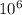 eV = 2.196 eV
eV = 2.196 eV



