
Chemistry, 07.12.2019 04:31, maxy7347go
In which solution(s) will a precipitate form? i. 0.00090 g of na2cro4 (molar mass = 162.0 g/mol) is added to 225 ml of 0.00025 m agno3. ksp ag2cro4 =1.1 x 10-12 ii. 0.100l of 0.0015 m mgcl2 and 0.200l of 0.012 m naf. ksp mgf2 = 3.7 x 10-8

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, 10040813
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3


Chemistry, 23.06.2019 01:00, tjeffers90028
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
Do you know the correct answer?
In which solution(s) will a precipitate form? i. 0.00090 g of na2cro4 (molar mass = 162.0 g/mol) is...
Questions in other subjects:



Mathematics, 20.05.2021 17:50

Mathematics, 20.05.2021 17:50


English, 20.05.2021 17:50


Mathematics, 20.05.2021 17:50

English, 20.05.2021 17:50

Mathematics, 20.05.2021 17:50


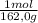 = 5,56x10⁻⁶ moles of Na₂CrO₄≡ moles of CrO₄²⁻.
= 5,56x10⁻⁶ moles of Na₂CrO₄≡ moles of CrO₄²⁻.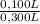 = 0,0005M MgCl₂≡ Mg²⁺
= 0,0005M MgCl₂≡ Mg²⁺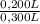 = 0,008M NaF ≡ F⁻
= 0,008M NaF ≡ F⁻



