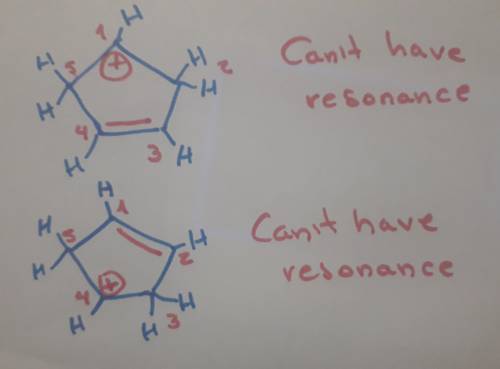
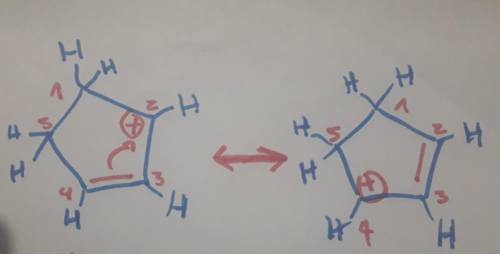
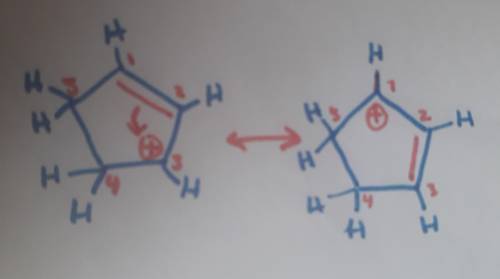
Carbons 1 and 4 of 1,3−cyclopentadiene are equivalent and give the same carbocation on protonation. likewise, carbons 2 and 3 are equivalent. write the structure of the carbocation formed by protonation of c−2 or c−3 to verify that it is not allylic and therefore not as stable as the one formed by protonation of c−1 or c−4.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 15:00, hockeykid7583
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 18:20, juansebas35
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
Do you know the correct answer?
Carbons 1 and 4 of 1,3−cyclopentadiene are equivalent and give the same carbocation on protonation....
Questions in other subjects:

Physics, 13.07.2019 17:30



Mathematics, 13.07.2019 17:30

History, 13.07.2019 17:30

Mathematics, 13.07.2019 17:30



Mathematics, 13.07.2019 17:30

Mathematics, 13.07.2019 17:30